Basketball Legend Pau Gasol joins Idoven in a new investment round. Read more here.
AI technology, based on science









Idoven has developed the world’s first cardiology-as-a-service platform powered by artificial intelligence that augments a clinician’s ability to identify, triage and diagnose patients at scale.
We are driving innovation and enhancing patient care across several areas of healthcare.

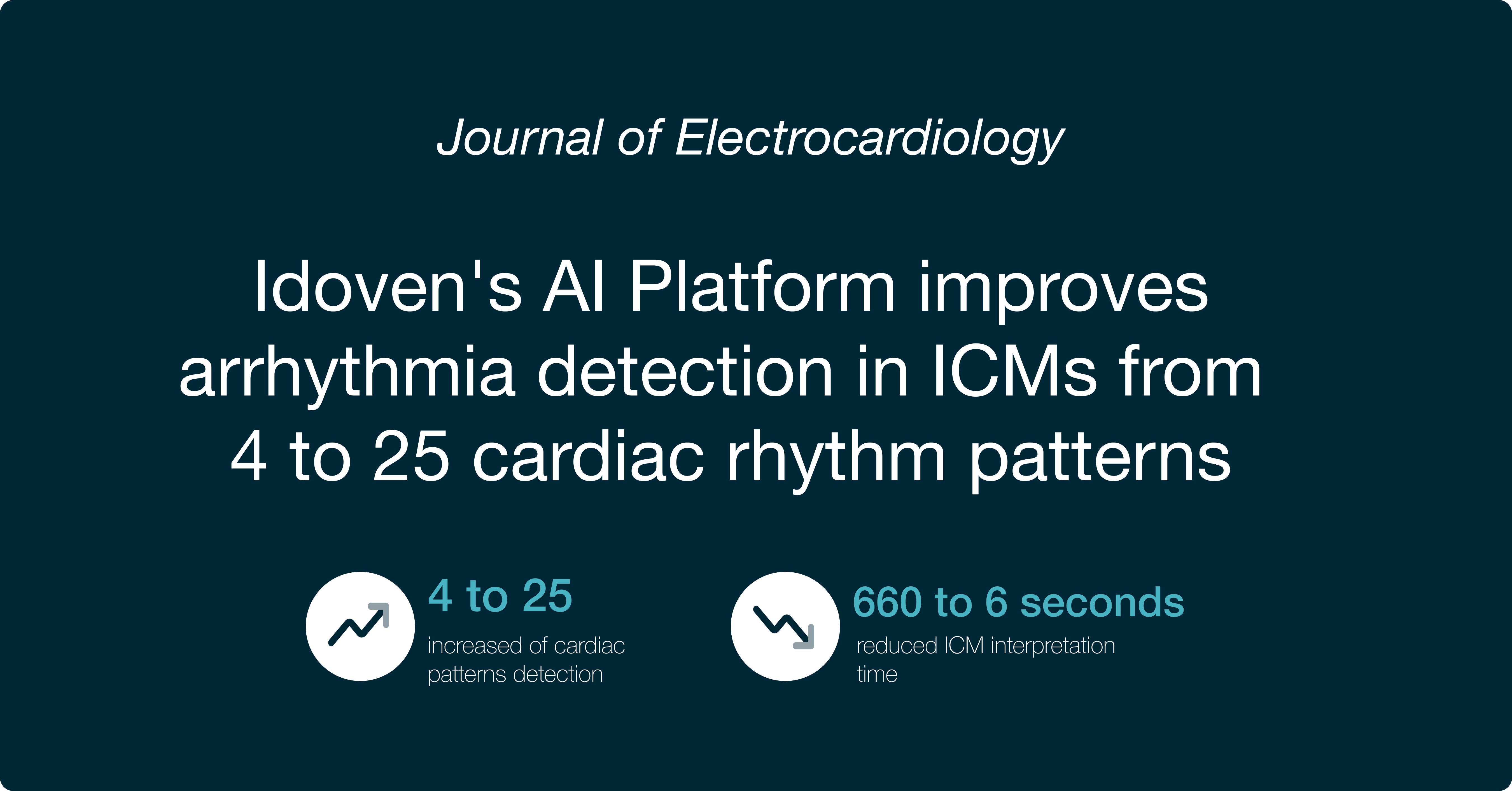
We enable rapid and accurate identification of arrhythmias and cardiac patterns to significantly reduce the time and expense associated with ECG interpretation.
Our solution can seamlessly integrate with Home Monitoring and Electronic Health Record (EHR) systems for efficient triage and diagnosis of patients at scale.

We develop disease risk scores using ECG-based biomarkers to identify high-risk, undiagnosed, and/or undertreated patients.
Our solution helps clinicians in the prediction of potential cardiovascular diseases to improve patient management and enhance treatment outcomes while monitoring cardiac drug safety.

We provide comprehensive preventative screening programs to help organisations better manage their employees’ health and wellbeing.
Our solution provides continuous remote ECG monitoring from non-invasive devices to anyone worldwide. Our proprietary algorithms and cardiologists analyse the data and deliver a medical-grade report.
ECG hours in database, manually annotated for the sole purpose of AI training

Patients monitored in clinical trials

Collaborators from leading EU and US research centres and industry partners on stroke and atrial fibrillation (MAESTRIA H2020)
Publications in top-tier scientific journals
Prediction models targeting cardiovascular disease under development
Cardiac patterns detected automatically with AI

%201.png)
Our cloud-based infrastructure provides reliability, compliance and security.


Ingests data from standard and proprietary formats.
Analyses ECGs of any duration, to deliver results in minutes.

Works with any ECG device compliant with standards IEC 60601-2-25, IEC 60601-2-27 and IEC 60601-2-47














.png)






.png)






.png)
We are working with leading pharmaceutical and medical device companies in 3 focus areas:


On May 1, 2019, Iker Casillas, World-cup winning goalkeeper and United Nations Goodwill ambassador, suffered a heart attack during training at the age of 38. Today, he relies on Idoven to care for his heart. Together with the Iker Casillas Foundation, Idoven is able to impact the lives of vulnerable groups at high risk of cardiovascular diseases.
Join Iker, olympic athletes and global tech organisations in the #donateyourheartbeats movement to advance research in the prevention of heart attacks, strokes and other cardiovascular diseases.
Idoven is at the very forefront of advancing AI technology in health and cardiovascular care to help doctors and patients all over the world. We'd like your help.



.png)